Ligand-protected metal (Au, Au, Cu) nanoclusters have been investigated extensively because of their unique physicochemical properties and applications in fields such as optics, catalysis, chemical sensing, and magnetism. A number of ligand-protected Au or Ag nanoclusters with phosphine or thiorate ligands have been synthesized and studied in detail. The properties of ligand-protected metal nanoclusters can be “tuned” by the choice of the protecting ligands as well as the size of the clusters (i.e., the number of Au atoms in the cluster core). The electronic and optical properties of metal nanoclusters depend strongly on the cluster core, while the surface ligand species affect the surface chemistry, for example, solubility and solution-phase properties. In some cases, the surface ligand also influences the fluorescence of metal nanoclusters. Our groups focus on synthesis of water-soluble metal (Au, Ag, Cu) nanoclusters and their applications such as photoluminescence, sensing, PDT Application, Antibiotic properties for Biomedical Applications, and Photo/Synthetic Catalysis.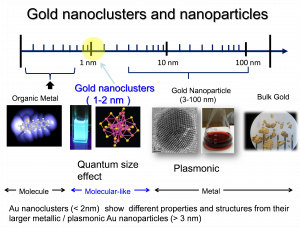

Photoluminescence and Sensing Application
・Kawasaki et al. Adv. Funct.Mater., 21, 3508(2011)
・Kawasaki et al. Anal.Sci., 27, 591(2011)
・Sakanaga and Inada* et al. Applied Physics Express, 4 (9), 095001(2011)
・Yoshimoto et al. Chem. Lett., 43, 793(2014)
・Chiba and Yonezawa* et al. J. Mater. Chem. C, 3, 514(2015)
・Yoshimoto and Iwasaki et al. J. Phy. Chem. C, 119 , 14319(2015)
・Saita et al. J. Lumin. 232, 117849(2021)
・Saita et al. Luminescence, 38,127(2023)
・Saita et al. Opt. Mater. 139,113803(2023)
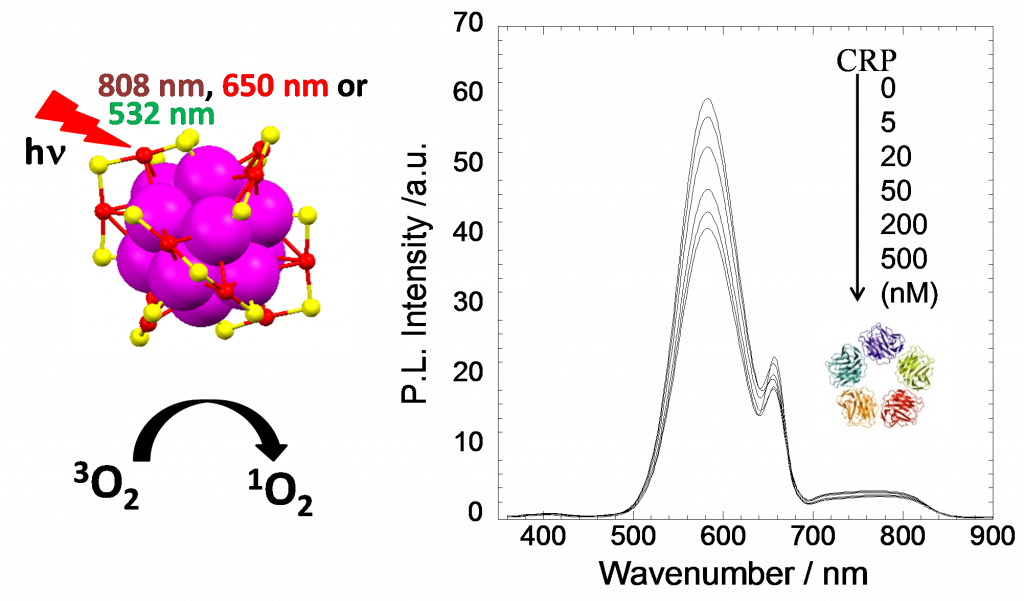
Photo/Sono-catalysis and PDT/SDT Application
・Changlin et al J. Phys. Chem. Lett., 4, 2847 (2013).
・Kawasaki et al., Chem. Mater., 26, 2777(2014)
・Yamanoto et al., J. Lumin., 180, 315-320(2016)
・Miyata and Miyaji* et al., Int. J. Nanomed. 12, 2703(2017)
・Miyata and Miyaji* et al., Biol. Eng. Med. (2017), doi: 10.15761/BEM.1000126
・ Yamamoto et al., J. Colloid. Interf. Sci. 510, 221(2018)
・Tominaga et al., Acta Physico-Chimica Sinica 34, 805 (2018)
・ Hikosou et al., J. Phys. Chem. C 122, 12494–12501(2018)
・Kawamura et al. J. Phys. Chem. C 123, 43, 26644(2019)
・[Review] Kawawaki et al., Nanoscale Advances 2, 17(2020)
・Shitomi et al., Photodiagn. Photodyn. Ther. 30, 101647(2020)
・Okamoto et al., ACS Omega 6, 9279(2021)
・Kawamura et al., J. Chem. Phys. 155, 124702(2021)
・Saita et al., Colloids and Surfaces A: Physicochemical and Engineering Aspects, 617, 126360(2021).
・Yagi et al., J. Phys. Chem. C, 126, 19693 (2022)
・Mori et al. J. Mater. Sci. 58, 2801 (2023)
・Yang et al., ACS Applied Bio Materials, 6, 4504(2023)
・Tsurunishi et al., Molecules, 30, 541 (2025).
Antibacterial properties for Biomedical Applications
・Tominaga et al. RSC Adv. 6, 73600(2016).
・Sangsuwan and Iwasaki* et al. Colloids and Surfaces B: Biointerfaces 140, 128(2016)
・Sangsuwan and Iwasaki* et al. Bioconjugate Chemistry 27 , 2527(2016)
・Tominaga et al. ACS Appl. Nano Mater. 1 , 4809(2018)
・Miyaji et al., Scientific Reports,12, 16721 (2022)
・Yang et al, Appl Mater Today, 40,102419(2024)
Catalysis
・Kawasaki et al. Langmuir 26 , 5926(2009)
・Yamamoto et al. Nanoscale 4 , 4148(2012)
・Kawasaki et al. Chem. Commun. 46, 3759(2010)
・Hyotanishi and Obora* et al. Chem. Commun. 47, 5750(2011)
・Isomura and Obora* et al. Chem. Commun. 48, 3784(2012)
・Sugii et al. J, Nanoparticle Res. 15, 1379(2013)
・[Review] Kawasaki, Nanotechnology Reviews 2 , 5(2013)
・Oikawa and Obora* et al. Chem. Commun. 53, 1080(2017)
・ Azuma, and Obora* et al. ChemCatChem.10, 2378 (2018)
・Kuroda et al. Colloid Interf. Sci. Commu. 31, 100187(2019)
・Kawasaki et al., Chem. Commun.,58, 7741(2022)
・Manyuan et al. RSC Advances, 13, 30273(2023)
・Manyuan et al. ACS Appl. Mater. Interfaces,17,6414(2025)
